
Reaction of benzene with halogens, chlorine, bromine, iodine

Reaction of benzene with halogens
This requires the use of electron carriers (AlCl3, FCl3) as catalysts. The electron carrier is usually a halide of iron or aluminium, the electron carriers’ function is to polarize the halogen molecule by withdrawing the electrons from the bond between the two halogen atoms.
Example
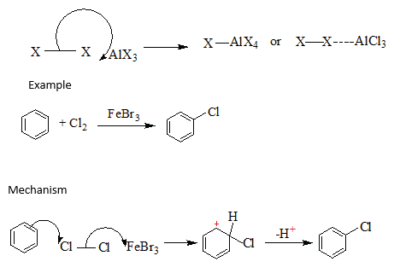
Chlorobenzene was historically important in the manufacture of chlorinated pesticides, especially DDT, and in the production of phenol and aniline. Monochlorobenzene’s principal current use is as a chemical intermediate in the production of chemicals such as nitrochlorobenzenes and diphenyl oxide. These chemicals are subsequently used in the production of herbicides, dyestuffs, and rubber chemicals. Additionally, monochlorobenzene is used as a solvent in degreasing processes (e.g., in metal cleaning operations), paints, adhesives,waxes and polishes.
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much

Your blog is a breath of fresh air. Health & Personal Care
Thanks for making this so enjoyable. 500 ka redeem code
Simplify the admission process with MBBS Direct Admission in Himachal Pradesh.
Pursue your dream career in healthcare at Top MBBS Colleges in Punjab.
Use the Raja Luck Invite Code to get exclusive rewards and bonuses.