
Respiration (A-level notes)

Respiration for A-level
This is the oxidation of organic substance to liberate energy in the body. Aerobic respiration requires oxygen whereas anaerobic respiration does not require oxygen.
Organic molecules (usually carbohydrate or fat) are broken down bond by bond, by a series of enzyme – controlled reactions. Each bond broken releases a small amount of energy converted to adenosine triphosphate (ATP). ATP is the immediate source of energy for cellular reactions.
Uses of ATP in the body
- Provide energy to build up macromolecules such as proteins from amino acids, polysaccharides form monosaccharides and DNA synthesis.
- Provide energy for movement of materials such as active transport.
- Provides energy for secretion of materials.
- Provide energy for muscle contraction, spindle formation in cell division and ciliary action
- Provide energy for activation of molecules before they are used in the body.
Cell respiration.
Cell respiration involves oxidation of a substrate to yield chemical energy (ATP). Organic compounds which are used as substrates in respiration are carbohydrates, fats and proteins.
Carbohydrates:
These are usually the first choice of most cells. In fact, brain cells of mammals cannot use anything but glucose. Polysaccharides are hydrolyzed to monosaccharides before they enter the respiratory pathway i.e. starch in plant and glycogen in animals are first converted to glucose.
Fats/lipids.
They form the “first” reserve and are mainly used when carbohydrate reserves have been exhausted. However, in skeleton muscle cells, if glucose and fatly acids are available, these cells respire the acids in preference to glucose.
Lipids are better energy source than carbohydrates because they have a higher proportion of hydrogen and an almost insignificant proportion of oxygen compared with carbohydrates. Thus a given mass of lipids yields more energy on oxidation than an equal mass of carbohydrate.
Proteins.
Since proteins have other essential functions they are only used when all carbohydrate and fat reserves have been used up, as during prolonged starvation.
The break of glucose in respiration.
When glucose is the substrate of respiration, its oxidation can be divided into three phases, glycolysis, oxidative de-carboxylation (Krebs or citric acid cycle or TCA, tri-carboxylic acid cycle); oxidative phosphorylation. Glycolysis is common to anaerobic and aerobic respiration, but the other two phases only occur only in presence of oxygen.

Glycolysis represents a series of reactions in which a glucose molecule is broken down into two molecules of pyruvate. Glycolysis occurs in the cytoplasm of cells, not in the mitochondria, and does not require the presence of oxygen. The process may be subdivided into two steps, first the conversion of glucose into fructose 1,6 – di-phosphate, and secondly the splitting of fructose -1,6- di-phosphate into 3C sugars which are later converted into pyruvate. Two ATP molecules are used up for phosphorylation reaction in the first step. Whilst four ATP molecules are produced in the second step. Four hydrogen atoms are also release. These are oxidized to water by molecular oxygen with accompanying phosphorylation of ADP to ATP molecules during oxidative. Each pair of hydrogen atom is oxidized to form 2 ATP
The ultimate fate of pyruvate depends on the availability of oxygen in the cell. If it is present, pyruvate will enter a mitochondrion and be completely oxidized into carbon dioxide and water aerobic respiration. If oxygen is unavailable pyruvate will be converted into ethanol or lactate (anaerobic respiration).
Summary
| source | Number of ATP |
| From stages 6 and 7 | 4ATP |
| ATP from NADH2 | 4ATP |
| Total | 8ATP |
Aerobic respiration.
There are two phases involved in aerobic respiration, first, if sufficient oxygen is available, each pyruvate molecule enters a mitochondrion where it is converted into a 2-carbon compound acetyl co-enzyme A (acetyl CoA for short). In this reaction carbon dioxide is given off, and the pyruvate loses a pair of hydrogen atoms which again results in the synthesis of ATP.
Acetyl CoA is a very important intermediate in respiration. It links glycolysis, oxidation of fats and proteins with the Krebs cycle.
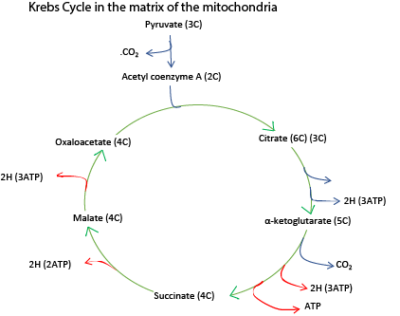
The Krebs cycle, tricarboxylic cycles or citric cycle
- Before pyruvate enters the Krebs cycle it combines with a compound called coenzyme A to form Acetyl coenzyme A. in the process, a molecule of carbon dioxide and a pair of hydrogen atoms are removed.
- The 2-carbon acetyl coenzyme A enters the Krebs cycle by combining with a 4-carbon oxaloacetate (oxaloacetic acid to give a 6-carbon citrate (citric acid). This reaction requires energy which is provided at the expense of the energy-rich bond of acetyl group CoA.
- The citrate is degraded to a 5-carbon α-ketoglutarate (α-ketoglutaric acid) and then the 4-carbon oxaloacetate by progressive loss of two carbon dioxide thus completing the cycle.
- For each turn of the cycle, a total of four pairs of hydrogen atoms are also formed.
- Of these, 3 pairs of hydrogen atoms are combined with hydrogen carrier, nicotinamide adenine dinucleotide (NAD) and yield three ATP for each pair of hydrogen atoms. The remaining pair of hydrogen atoms combine with a different hydrogen carrier, Flavine adenine dinucleotide (FAD). And yield 2 ATP.
- It must be remembered that all these products are formed from a single pyruvate molecule of which two are produced from each glucose molecule.
Summary
| Source of ATP | Number of ATP | Total number |
| 3 pairs if H with NAD | 9ATP | 9ATP x 2 |
| 1 pair of H with FAD | 2ATP | 2ATP x 2 |
| Energy of the cycle | 1ATP | 1ATP x 2 |
| Glycolysis process | 8ATP | 8ATP x 1 |
| Pyruvate to Acetyl Co A | 3ATP | 3ATP x 2 |
| Total | 38 ATP | |
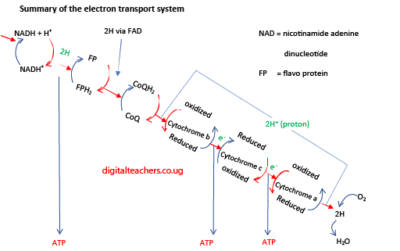
Electron transport in the stalked membrane of the mitochondria
It occurs in the inner membranes of the mitochondria. It is the means in by which the energy, in the form of hydrogen atom, from Krebs cycle, is converted to ATP. The hydrogen atoms attached to a hydrogen carriers NAD and FAD are transferred to a chain of other carriers at progressively lower energy levels. As the hydrogens pass from one carrier to the next, the energy released is used to produce ATP. The series of carrier is called respiration chain. The carriers in the chain include NAD, Flavo protein, coenzyme Q and iron-containing protein called cytochromes. Initially hydrogen atoms are passed along the chain, but latter split into their proton and electron and only the electron pass from carrier to carrier. For this reason, the pathway can be called electron or hydrogen transport system. At the end of the chain the protons and electrons recombine, and the hydrogen atoms create a link with oxygen to form water. This formation of ATP through the oxidation of the hydrogen atoms is called oxidative phosphorylation. The role of oxygen is to act as the final electron acceptor.

Function of mitochondria
Production of cellular energy, ATP
Adaptationsof mitochondria
- Has extensive inner membrane to create a high surface area for enzymes that produce.
- have a small amount of mitochondrial DNA, allowing them to create mitochondrial proteins quicker than from nucleic genes.
- Have necessary enzymes for production of energy
Anaerobic respiration
A variety of microorganism (anaerobes) employ anaerobic respiration as their major ATP yielding process. Organism that survive only in absence of oxygen are termed obligate anaerobes e.g. C. Brotulium and C. tetani). Obligate anaerobe find oxygen poisonous.
Other organisms such as yeast and alimentary canal parasites (such as tape worms), can exist whether oxygen is available or not. These are called facultative anaerobe. Also, some cells that are temporarily deprived of with no oxygen available to accept the hydrogen oxygen (such as muscle cells) are able to respire anaerobically.
Pyruvate serves as an electron/hydrogen acceptor in absence of oxygen to accept the hydrogen atoms released during glycolysis; and depending on the metabolic pathways within the organisms’ cells, the end-product of anaerobic respiration will either be ethanol and carbon dioxide (e.g. fermentation as in yeast) or lactate, for example lactate fermentation in muscle cells:
In Alcoholic fermentation the glucose is converted to ethanol and carbon dioxide

Alcoholic fermentation is the basis of brewing in which ethanol is an important product and baking industry in which carbon dioxide expands the dough.
Lactic fermentation occurs occasionally in animal cells during strenuous exercise and oxygen is insufficient. It allows animal to survive periods of insufficient oxygen. When oxygen is latter availed, lactic acid is oxidized to carbon dioxide and water or can be turned into carbohydrates. The amount of oxygen required to oxidize lactic acid accumulated in muscles is called the oxygen debt.
Differences in aerobic and anaerobic respiration
| Aerobic Respiration | Anaerobic Respiration | |
| Definition | Aerobic respiration uses oxygen. | Anaerobic respiration is respiration without oxygen; the process uses a respiratory electron transport chain but does not use oxygen as the electron acceptors. |
| Cells that use it | Aerobic respiration occurs in most cells. | Anaerobic respiration occurs mostly in prokaryotes |
| Amount of energy released | High (36-38 ATP molecules) | Lower (Between 36-2 ATP molecules) |
| Stages | Glycolysis, Krebs cycle, Electron Transport Chain | Glycolysis, Krebs cycle, Electron Transport Chain |
| Products | Carbon dioxide, water, ATP | Carbon dixoide, reduced species, ATP |
| Site of reactions | Cytoplasm and mitochondria | Cytoplasm and mitochondria |
| Reactants | glucose, oxygen | glucose, electron acceptor (not oxygen) |
| combustion | complete | incomplete |
| Production of Ethanol or Lactic Acid | Does not produce ethanol or lactic acid | Produce ethanol or lactic acid |
Alternative respiratory substances
- Fats are oxidized after hydrolysis to glycerol and fatty acids. Glycerol is phosphorylated, and converted into glycealdehyde-3-phosphate which is incorporated into the glycolysis pathway. Fats produce more hydrogens than glucose/carbohydrates and hence produce more energy.
- Proteins are used only in cases of starvation. They are hydrolyzed to constituent amino acids which are deaminated to form carbohydrates before converted to pyruvate.
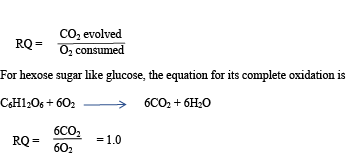
Respiratory Quotients
The respiratory quotient (RQ) is the measure of the ratio of carbon dioxide evolved by an organism to the oxygen consumed, over a certain period.
Importance:
(i) Knowledge of respiratory quotient helps in determining respiratory substrate.
(ii) It helps in knowing the type of respiration being performed,
(iii) It provides some information about major transformation of food materials.
In fats and proteins, the ratio of carbon dioxide evolved to oxygen consumed is far smaller than in carbohydrates. A fat and proteins, therefore requires more oxygen for its complete oxidation than carbohydrates. The respiratory quotient of fats is 0.7 whereas that of proteins is 0.9. Organisms rarely respire one substance at a time, thus the respiratory quotients of an animal is between 0.8 and 0.9.
Low respiratory quotient may also indicate that carbon dioxide produce is used elsewhere for example;
(i) at compensation point during photosynthesis carbon dioxide produced used is in photosynthesis
(ii) during egg formation carbon dioxide produced is used for shell formation. Carbon dioxide is the source of carbonate ions for formation of CaCO3 in egg shells
(iii) RQ is less than one when respiration is aerobic but the respiratory substrate is either fat or protein. RQ is about 0.7 for most of the common fats. It occurs during germination of fatty seeds.

(iv) RQ Zero:
Succulents do not evolve carbon dioxide during night (when their stomata are open) as the same is used in carbon fixation. They also change carbohydrates to organic acids which utilise oxygen but do not evolve carbon dioxide.
2C6H12O6 + 3O2 → 3C4H6O5 + 3H2O
RQ = CO2/3O2 = Zero/infinity
High respiratory quotients occur
- When carbohydrates are converted into fats. when an animal is preparing to hibernate and in fattening livestock since during conversion of carbohydrates to fat carbon dioxide is liberated when pyruvate is converted Acetyl CoA. Acetyl CoA is then converted to fats without using oxygen
- During anaerobic respiration because carbon dioxide is given out, but no oxygen used.
Basal metabolic rate
Is the minimal rate of energy expenditure per unit time by endothermic animals at rest.
Factors affecting the BMR
- Body size: Metabolic rate increases as weight, height, and surface area increase.
- Body composition: Fat tissue has a lower metabolic activity than muscle tissue. As lean muscle mass increases, metabolic rate increases.
- Gender: The basal metabolic rate (BMR) averages 5 to 10 percent lower in women than in men. This is largely because women generally possess more body fat and less muscle mass than men of similar size.
- Age: A decrease in lean muscle mass during adulthood results in a slow, steady decline of roughly 0 3 percent per year in BMR after the age of about 30. This can be largely avoided by strength training throughout adulthood.
- Climate and body temperature: The BMR of people in tropical climates is generally 5 to 20 percent higher than their counterparts living in more temperate areas because it takes energy to keep the body cool. Exercise performed in hot weather also imposes an additional metabolic load. Body fat content and effectiveness of clothing determine the magnitude of increase in energy metabolism in cold environments; it takes energy to keep the body warm if you work or exercise in very cold weather.
- Hormonal levels: Thyroxine (T4), the key hormone released by the thyroid glands has a significant effect upon metabolic rate. Hypothyroidism is relatively common, especially in women near or after menopause. Everyone with a weight problem should have their thyroid function checked by their doctor and treated appropriately if it turns out to be low.
- Health: Fever, illness, or injury may increase resting metabolic rate two-fold
Please find free downloadable notes, exams and marking guides of agriculture, biology, Physics, chemistry etc. from digitalteachers.co.ug website.
Dr. Bbosa Science

I’m always impressed by your writing. Toys & Games
I appreciate the time you put into this. Transfer Latest
Ensure your future with MBBS Admission Through Management/Nri Quota in Madhya Pradesh.
MBBS Admission Through Management/Nri Quota in West Bengal offers a seamless pathway to education.
Get the latest Raja Luck App Download for a seamless gaming experience.