
Sketch a pressure versus volume curve for a real gas undergoing compression

Sketch a pressure versus volume curve for a real gas undergoing compression
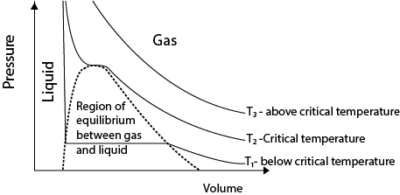
- Above the critical temperature, a real gas behaves ideally.
- Below the critical temperature the turns into a liquid on compression as shown.
- A liquid is not compressible that is why pressure increases very rapidly with a small decrease in volume.
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES heat A-level
TAGS Dr

I always leave your blog feeling inspired. Industrial & Scientific
I’m always excited to see what’s next. Tech News
Explore seamless opportunities with MBBS Direct Admission in Chhattisgarh.
Discover how to fast-track your education through MBBS Direct Admission in Bihar.
Download the Raja Luck App to enjoy seamless gaming on your mobile device.
Access Goa Games across multiple devices, allowing you to play anytime, anywhere, without compromising on quality.
Want to rank faster? Get Backlinks for My Website and enhance your SEO results today.
Release high-performance computing applications with Server Rental in Mumbai.
Register on 55-club and dive into a world of competitive video gaming.
Establish a strategic IT Strategy Roadmap to drive service innovation and performance.
I was impressed by how fast OnlyMP3 converts videos to MP3 with no loss in quality.
Terrific suggestions for dining areas! I enjoyed them more with company set up through Escort Service In Nainital during my check out.
Uttarakhand Cuisine is a concealed gem in Indian food culture.