
Structure of sodium chloride

The sodium chloride structure
The x-ray analysis of crystal structures show that sodium chloride consists of a regular three-dimensional assembly of Na+ and Cl– ions.
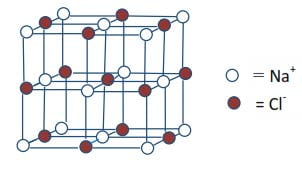
Each sodium ion is surrounded by six Cl– ions as its neighbors and each Cl– is surrounded by six Na+ ions. Therefore, both the sodium ion and chloride ions in the structure have a coordination number of six. Sodium chloride is an ionic compound that conducts electricity in molten and solution forms.
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES Inorganic chemistry questions
TAGS Dr. Bbosa Science
