
Thermodynamics (A-level physical chemistry)

Thermodynamics (A-level)
Deals with energy changes in chemical reactions
In most chemical reactions there is an associated energy change, shown by the evolution of heat in an exothermic reaction /process, or by absorption of heat in an endothermic reaction.
It is generally possible to measure what the change is, even though it is may not be possible to determine the absolute energy levels before and after the change.
Heat changes at constant volume are known as internal energy change (∆U) while those at constant pressure are known as enthalpy changes (∆H). If no volume changes are involved ∆H = ∆U, but with a volume change, the difference between ∆H and ∆U arises because work is involved in expansion or contraction. For practical purposes, these heat changes are used interchangeably.
Heat absorbed or liberated in a reaction varies with the quantities of the reactants, the temperature, and the pressure at which a reaction is carried out.
Standard enthalpy changes
These are enthalpy changes when molar quantities are considered at 298K and 1 atmospheres.
Standard molar enthalpy changes are then symbolized by ∆HmӨ(298K) but the simpler symbol, ∆H, may be used. ∆H has a negative value (heat evolved) for exothermic reaction and positive value for an endothermic reaction.
The terms used for the different energy changes are as follows:
Enthalpy of combustion
The standard molar enthalpy of combustion of a substance is the enthalpy changes when 1 mole of it is completely burned in oxygen under standard conditions.
E.g.
∆Hc,mӨ(298K)
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) – 890.5kJmol-1
C (s) + O2(g) → CO2(g) – 393.5kJmol-1
(i) An experimental method for finding enthalpy of combustion a liquid fuel
The figure below shows a simple method for obtaining approximate value for the enthalpy of combustion of a fuel
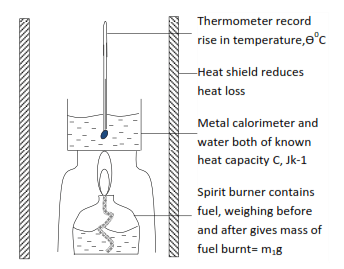
Calculations
Assumption
The heat produced by combusting fuel = heat gained by the water
Heat gained by calorimeter and water = CӨ joules
It implies that
m1g of fuel produce = CӨ joules
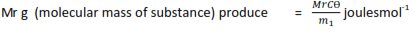
(ii) An experimental method for finding enthalpy of combustion using a calorimetric bomb.
This method is suitable for the determination of enthalpy of combustion of solid fuels such as carbon, Sulphur or sugar but can be used on a liquid fuel
Apparatus

Procedure
1. A known mass, m1 g, of a substance is placed in a platinum crucible, C.
2. The bomb is filled with oxygen at a pressure of about 20atmospheres and immersed in a known mass (mw g) of water which is kept stirred.
3. The substance is ignited by passing an electric current through a small coil of iron wire, W.
4. The rise in temperature (θ) of water is read to 0.010C with an accurate thermometer.
Calculation
Heat produced by burning m1 g of substance = heat gained by mw g of water
= mw x 4.2 x θ
(where 4.2 is the specific heat capacity of water)

Where Mr is the relative molecular mass of the substance.
Enthalpy of formation of compounds:
The standard molar enthalpy of formation of a compound is the enthalpy change when 1 mole of the compound is formed from its elements under the standard conditions. e.g.
H2(g) + ½ O2 →H2O(l) ∆Hf,mӨ(298K) = -285.9kJmol-1
As there is no energy change information of an element under standard conditions; the standard enthalpies of all pure elements in their standard states is zero
Enthalpy of formation of hydrated ions;
This refers to enthalpy change of the formations of 1mole of hydrated ions from an element in its standard state. e.g.
Na(s) + aq → Na+(aq) + e ∆Hf,mӨ(298K) = -240kJmol-1
The figures are usually quoted on an arbitrary scale on which the enthalpy of formation of H+(aq) is taken to be zero. i.e.
½ H2(g) → H+(aq) + e ∆Hf,mӨ(298K) = 0kJmol-1
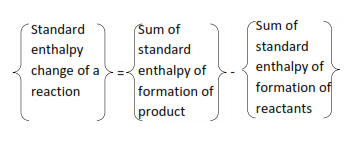
Enthalpy of reaction
This is the enthalpy change when 1 mole of a substance is formed from its reactants at standard conditions. Tabulated values of the standard enthalpies of formation of compounds and or hydrated ions can be used to calculate the standard enthalpy change for any reaction
Typical examples
NH3(g) + HCl(g) → NH4Cl(s)
(-46) (-92.3) (-315)
∆Hr,mӨ(298K) = -315 – (-46 + -92.3) = -177kJmol-1
The negative sign means that reaction is exothermic.
The standard enthalpy depends only on the difference between the standard enthalpy of the reactants and the product and not on the route by which the reaction occurs.
The ideal is embodied in Hess’s law, which states that “if a reaction take place by more than one route, the overall change in enthalpy is the same whichever route is followed” or “enthalpy change of a reaction is independent of the route followed to the product”.
Enthalpy of atomization
Refers to the enthalpy change for the formation of 1 mole of gaseous atoms from an element in its standard state
C(s)(graphite) → C(g) ∆Ha,mӨ(298K) = +715kJmol-1
½ H2(g) → H(g) ∆Ha,mӨ(298K) = +218kJmol-1
Enthalpy of ionization
refers to enthalpy change for the conversion of gaseous atoms into free gaseous ions, e.g.
Na(g) → Na+(g)+ e ∆HmӨ(298K) = +502kJmol-1
Factors affecting the magnitude of ionization energy
- Size of an atom: Small atoms have a strong attraction to outer electrons leading to high ionization energy.
- Electronegativity: highly electronegative atoms have a high attraction to valence electron causing high ionization energy.
- Electropositivity: highly electropositive atoms have low attractions to valence electrons leading low ionization energy.
Enthalpy of electron affinity
refers to the enthalpy change for the formation of free gaseous anions from 1 mole of free gaseous atoms.
Cl(g) + e → Cl– (g) ∆HmӨ(298K) = -354kJmol-1
Factors affecting the magnitude of electron affinity
- Size of an atom: Small atoms have a strong attraction to outer electrons leading to high negative electron affinity.
- Electronegativity: highly electronegative atoms have a high attraction to valence electron causing high negative electron affinity
- Electropositivity: highly electropositive atoms have low attraction to valence electrons leading high positive electron affinity.
Lattice enthalpy
This is the name given to the enthalpy change for the reaction in which 1mole of crystalline solid is formed from its component ions in the gaseous phase.
Na+(g) + Cl-(g) → NaCl(s) ∆HmӨ(298K) = -788 kJmol-1
Factors affecting the magnitude of lattice energy
- Size of the ion; small ions have strong interionic attraction causing high lattice energy
- Charge on the ions: ions with big charge have strong interionic attraction leading to high lattice energy.
Enthalpy of solution
This refers to the enthalpy change for the formation of an infinitely dilute solution of 1 mole of salt.
NaCl(s) +aq→ Na+(aq) + Cl– (aq) ∆HmӨ(298K) = +4 kJmol-1
Factors affecting the magnitude of enthalpy of solution
Enthalpy of solution = lattice energy (+ve) + sum of hydration energy of ions (-ve)
When the lattice energy exceeds the sum of hydration energies of the ions, the enthalpy of solution is positive (endothermic) and the salt is insoluble.
When the lattice energy is less than the sum of hydration energies of the ions, the enthalpy of solution is negative (exothermic) and the salt is soluble.
Enthalpy of hydration
This refers to the enthalpy change when 1mole of gaseous ions is hydrated. e.g.
Na+(g) + aq → Na+(aq) ∆HmӨ(298K) = -406 kJmol-1
Factors affecting the magnitude of lattice energy
- Size of the ion: small ions have a strong attraction to water molecules leading to high hydration energy.
- Charge on the ions: ions with high big charge have a strong attraction to water molecules leading to the high enthalpy of hydration.
Enthalpy of neutralization
This refers to enthalpy change for the formation 1 mole of water is formed from hydrogen and hydroxide ions
H+(g) + –OH(aq) → H2O(l)
Measurement of standard enthalpy of neutralization
The heat released when a known amount of water is formed is found by measuring the temperature produced in a calorimeter and its contents.
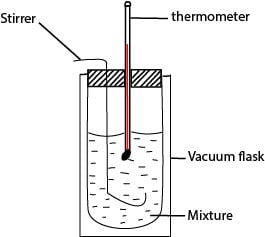
A neutralization reaction is carried out in the calorimeter of known heat capacity, C.
A known volume of standard acid (v cm3) and alkali (v cm3) are added to the calorimeter, and temperature change θ0C is noted.
The number of moles of water formed, X moles is calculated
Calculations
Assumptions:
- The density of water = density of solution = 1gcm-3.
- Specific heat of solution = specific heat capacity of water = c Jg-1K-1
- Heat is given out on neutralization = Heat received by water + calorimeter of capacity, C.

Example
250 cm3 of 0.40M were added to 250cm3 of 0.40M HCl in the calorimeter heat capacity of 90 J K-1. The temperature of the two solutions was 17.50C and rose to 20.10C
Calculate the enthalpy of neutralization assuming that the specific heat capacities of the solution are the same as that of water = 4.180J g-1 K-1.
Calculation
Temperature change θ = 20.1 – 17.5 = 2.60C
Heat liberated = (Cθ + 2vcθ)J
= 90 x 2.6 + (250 + 250) x 4.180 x 2.6)
= 5668J

Enthalpy diagrams
These are constructed by the application of Hess’s law: that states “enthalpy change of a reaction is independent of the route followed to the product.” For instance, the enthalpy change for conversion of carbon into carbon dioxide is the same whether it is carried out in one step;
C(s) + O2(g) → CO2 (g) ΔHmѲ(298K) = -393.5 kJ mol-1
Or two steps
C(s) + ½ O2(g) → CO (g) ΔHmѲ(298K) = -110.5 kJ mol-1
CO(s) + ½ O2(g) → CO2 (g) ΔHmѲ(298K) = -283.0 kJ mol-1
The relationship between various enthalpy changes is conveniently summarized in an enthalpy diagram below.

Such diagrams are used to obtain values for enthalpy changes that cannot be measured directly. The enthalpy of formation of methane cannot be measured directly since carbon and hydrogen do not react for example;
C(s) + 4H2 (g) → CH4(g) ΔHmѲ(298K) =x kJ mol-1
The enthalpies of combustion of carbon (-393.5 kJ mol-1), hydrogen (-289.9 kJ mol-1) and methane (-890.5 kJ mol-1) can, however, be measured and the required enthalpy of formation is obtained from them
(x = 74.8kJmol-1) as summarized in the figure below:
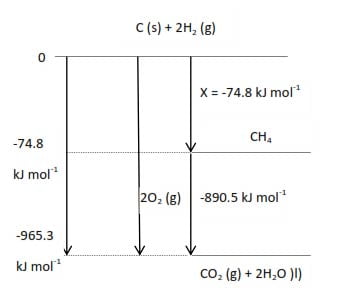
Standard enthalpy for a reaction from average standard bond enthalpies
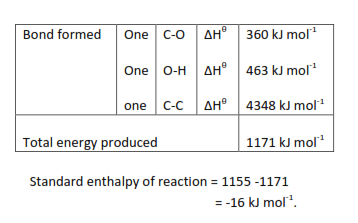
When the standard enthalpy for a reaction cannot be measured, an approximate value can be obtained by using average standard bond enthalpies. During reaction energy must be supplied to break bonds in the reactants and energy is given out when the bonds in the product form.
| The standard enthalpy of reaction | = | the sum of the average bond of reactants | – | sum of the average bond of products |

Watch these videos
For revision questions and answers download PDF

It’s very effortles too find ouut anny topic onn wweb as
compared to books, as I found thbis pieche off writing att ths web
site.
Thanks for making this topic accessible. Home & Kitchen
You have a real knack for this. Transfer Latest