
Vapour pressure, Raoult’s law and fractional distillation (A-level physical chemistry)

Vapor Pressure
All liquids exhibit tendency for evaporation at their surface. For evaporation to occur molecules at the surface acquire kinetic energy of liquid molecules overcomes the intermolecular force of attraction in the liquid state.
When evaporation is in a closed container system, the vapors molecules of liquid remain in contact with the surface of the liquid. Like gas molecules, vapor molecules also execute continuous random motion. During these motions, molecules collide with each other and also with the walls of the container, losses their energy and return back to a liquid state. This process is called as ‘condensation’.
Evaporation and condensation are continuous processes. Hence, after some time equilibrium is established, at a constant temperature between the rate of evaporation and rate condensation. At equilibrium number of molecules in vapor state remains constant at a constant temperature.
“The pressure exerted by vapors of liquid on the surface of liquid when equilibrium is established between liquid and its vapor is called VAPOR PRESSURE or SATURATED VAPOR pressure of the liquid.
The vapor pressure increases with temperature. When the saturated vapor pressure equals the external pressure the liquid boils, thus lowering pressure lowers the boiling point of a liquid whereas increasing external pressure increases the boiling points of liquids.
The former principle is employed during distillation of at reduced pressure (distillation in a vacuum) to purify substances which would decompose at their normal boiling points.
A plot of the saturated vapor pressure against temperature is shown below


t1 = boiling point at reduced pressure
t2 = boiling point at 1 atmosphere
t3 = boiling point at external pressure above 1amosphere
Measuring the saturated vapor pressure of a liquid
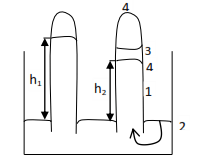

- a mercury barometer is set up
- Some liquid is introduced into the barometer
- Liquid float to the top of the mercury
- Vapor is formed. The vapor exerts pressure on the column of mercury
- The level of mercury drops. The difference (h1-h2) mm is the saturated vapor pressure of the liquid at this temperature.
The saturated vapor pressure of a liquid can be used to calculate the mass of the liquid that is present in vapor at a stated temperature
Example 1
A 1.00dm3 contains air at 1.01×105Nm-2 at 00C. After 1.000g of water is introduced into the vessel, the temperature is raised to 900C. Calculate the mass of water that will vaporize. The saturated vapor pressure of water at 900C is 6.99 x 104Nm-2.
Method
Use the ideal gas equation
PV = nRT
Substitute
P= 6.99 x 104Nm-2
R = 8.314JK-1mol-1
T = 363K
V = 1.000dm3 = 1.000 x 10-3m3
- x 104 x 1.000 x 10-3 = n x 8.314 x363
Moles of water vapor, n = 2.32 x10-2 moles
Mass of water vapor = 2.32 x10-2 x 18 = 0.417g
Effect of a solute on the vapor pressure of solvent
(a) Nonvolatile solute
This lowers the vapor pressure of a solvent
Reason
The presence of the particles of the nonvolatile solute reduces the escaping tendency of the solvent molecules from the surface of the liquid. That is, in the presence of the solute molecules, the probability of the solvent molecules to escape into vapor decreases. Therefore, the vapor above the solution and the vapor pressure of the solution are reduced
The vapor pressure versus temperature diagram for solvent and solution


The diagram indicates that nonvolatile solute raises the boiling point of the solvent.
Explanation
The presence of nonvolatile solute reduces the vapor pressure of the solvent and therefore, the solution must be heated to a higher temperature than the boiling point of the solvent to make its vapor pressure equals that of the atmosphere.
Raoult’s law of relative lowering of vapor pressure
Raoult’s law states that the relative lowering of the vapor pressure of a solution containing a nonvolatile solute is equal to the mole fraction of the solute in the solution.
If the vapor pressure of the pure solvent at a given temperature, t = P


where n1 = moles of solvent; n2 = moles of solute and is the mole fraction of solvent.
(b) Volatile solute
Here, both the solute and solvent are volatile and therefore each vaporizes and contributes to the vapor pressure above the solution. However, the more volatile component will contribute more to the vapor pressure than the other. Each component lowers the vapor pressure of the other.
Raoult’s Law
The partial pressure of any component at a given temperature in an ideal mixture varies linearly with its concentration.
Or
The partial vapor pressure of any component in a mixture at a given temperature is the product of its mole fraction in solution and the vapor pressure of the pure form at that temperature.
or

Consider a solution containing two components A and B and the number of moles of A and B are a and b respectively

According to Raoult’s Law
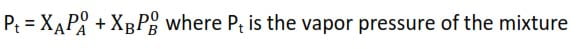
The total saturate vapor pressure is the sum of the partial pressure.
Definition
Partial pressure is the pressure that would be exerted by a component if it alone occupied by the volume occupied by the mixture.
According to Raoult’s Law
Ideal solution
An ideal solution is a solution that obeys Raoult’s law.
Definition
Partial pressure is the pressure that would be exerted by a component if it alone occupied by the volume of the mixture.
Properties of ideal solution
- the interactions between unlike molecules must are of the same magnitude as those between like molecules.
- The formation of an ideal solution does not involve a change in volume nor temperature.
Ideal solutions are often obtained by mixing two or more nonpolar solvents, for example, Heptane and octane.
A component that has a higher vapor pressure at a given temperature is said to be more volatile than a component with a low vapor pressure at the same temperature.
A plot of the vapor pressure against composition gives a vapor pressure composition curve.
The vapor pressure composition diagram for an Ideal mixture (A more volatile than B)

Composition of the vapor
The composition of the mixture above an ideal solution is obtained from Dalton’s Law that states that in an ideal mixture of gases that do not react chemically, the total pressure of the mixture is the sum of the partial pressures of the constituent gases.

Example 3
(a) State Raoult’s Law
(b) A mixture of liquids A and B obey Raoult’s Law. The vapor pressure of A and B are 10.00kNm-2 and 2.92kNm-2 respectively at 200C
Calculate the composition of the vapor of a mixture containing 0.5 moles of each liquid at 200C (3marks)

(b) Which of the liquids is more volatile? Give a reason for your answer
A is more volatile because has a higher vapor pressure than B at the same temperature.
Raoult’s Law and fractional distillation
When the vapor pressure of a liquid equates atmospheric pressure, the liquid boils.
Liquid or mixtures with high vapor pressure have lower boiling points than liquids or mixtures with lower vapor pressure. Actually, liquids with low vapor pressure have to be heated to higher temperatures in order to bring their vapor pressures equals atmospheric pressure to boil.
Consider two components A and B

Suppose A and B are mixed such that, the solution contains 20% A at a fixed temperature and we assume Ideal behavior.

It is observed that the vapor contains more of the more volatile component A than the less volatile component B compared to what was contained in the liquid mixture.
If the vapor is condensed, the distillate will have the same composition as the vapor and its total vapor pressure will be

=
Composition of distillate II will have the same composition as the vapor above
Again if this vapor is condensed into distillate III, the composition of the vapor of the distillate will be

The composition of the more volatile component keeps on increasing on every successive distillation; and if the procedure is repeated a number of times, time comes when the vapor contains only those of A (the more volatile component) hence complete separation of A from B. The residue will contain B
In practice, it is more convenient to perform distillation at constant pressure rather than constant temperature. The various steps in the distillation are better illustrated on the diagram below
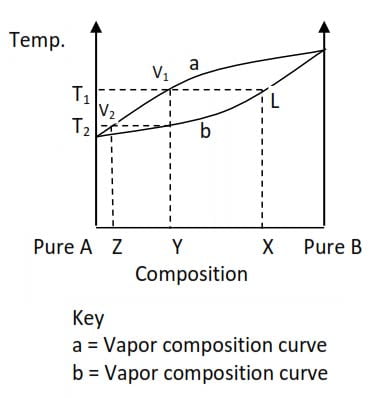
The lower curve shows the variation of the boiling point of the liquid as the distillation progresses.
Suppose the mixture to be distilled has composition X (20%A, 80%B) this mixture is represented by L on the diagram above.
The boiling of this liquid commences at a temperature T1. The vapor given off at T1 has a composition represented by V1.
When this vapor is condensed, the resultant liquid (composition Y) will be richer in the more volatile component A, and thus boils off at a lower temperature T2.
The vapor given off at this temperature has composition V2. If this vapor is condensed a liquid of composition Z is obtained.
By repeating the process, a sufficient number of times, we can obtain pure A. i.e. we can achieve complete separation of components
The number of distillation required to obtain the two pure components from equal proportions of the two components depends on the differences in their boiling points.
Such a separation of two completely miscible liquids by utilization of differences in their boiling points is known as fraction distillation.
However, the method just described above is slow and yield only small amounts of the pure components.
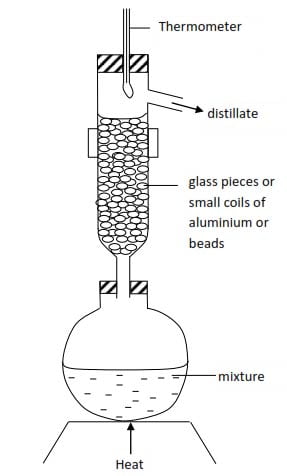
In practice, fractional distillation is carried out efficiently at constant pressure rather than at constant temperature with the help of a fractionating column (see below). In this case re-distillations are combined in one operation.
During distillation the temperature decreases progressively from bottom to top of the column and the ascending vapor is partially condensed.
Condensation of the vapor is effected by the large surface area of the glass pieces or beads and also by the liquid already condensed.
The vapor of the less volatile component condenses more readily than those of the more volatile component and return into the flask.
Therefore, the higher the vapor ascends in the column the richer it is in the more volatile component in the end, the two components will be able to separate.
Fractionating column
These are various forms and one of them is shown below
Example 4
The diagram below shows the boiling point composition of a mixture of liquid X and Y.

(i) Identify curves P and Q and points M and N (4 marks)
P– Vapor composition curve
Q– Liquid composition curve
M– Boiling point of Y
N– Boiling point of X
(ii) Which of the liquids is more volatile? Give a reason for your answers
Y because it has lower boiling point and hence high vapor pressure.
(iii) Describe what happens when the liquid of composition Z is boiled (4 marks))
The vapor coming off will contain more Y and the residue will contain a higher proportion of X
(iv) Explain how the principle in (iii) can be used to separate liquid mixtures by fractional distillation
If the vapor from (iii) is condensed, a liquid that contains more Y than in a liquid of composition Z is obtained.
Evaporating the distillate in (iii) gives a second distillate which is even richer in Y, than the distillate in (iii). This process of boiling a liquid of any composition and subsequent condensation of the vapor eventually give a vapor and hence a distillate of pure volatile component Y, the residue in the distillation flask will be the less volatile component X.
Deviations from Raoult’s law:
A solution is said to deviate from Raoult’s law when the adhesive and cohesive forces of attraction are not uniform between the two liquids molecule; consequently its vapor pressure (actual) differs from that calculated using Raoult’s law (ideal condition).
Positive deviation from Raoult’s law
Positive deviation from ideality is said to occur when the vapor pressure of the mixture is higher than what it would be from calculations of Raoult’s law.

Causes of positive deviation
Unlike molecules interact (attract) with one another less strongly than like molecules do.
i.e A↔B < A↔A or B↔B
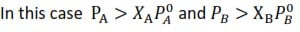
and therefore

Vapor pressure /composition diagram for a solution which deviates positively (A more volatile than B)

NB: maximum in vapor pressure forms towards the more volatile component.
Boiling point versus composition for a mixture that deviates positively at constant pressure

A maximum in vapor pressure produces a minimum in boiling point. When such a mixture that shows a positive deviation from ideality is distilled, a minimum in boiling point will be observed at certain compositions, and at this point the mixture has the same composition as the vapor above it.
And the further separation of the mixture into either component will be impossible.
The mixture corresponding to this minimum boiling point is called azeotrope and the corresponding temperature is called azeotropic temperature.
Definition
An azeotrope is a mixture that has the same composition as it vapors and boils at a constant temperature.
NB. Mixtures that deviate positively from Ideality are often obtained by mixing a polar and a non-polar solvent, for example, methanol, and cyclohexane. It is also observed that the azeotrope has a higher composition of the most volatile component.
Examples of solution that show positive deviation are:-
(a) Benzene and methanol
(b) carbon disulphide and ethanol
(c) chloroform and ethanol
The formation of a solution that deviates positively from Raoult’s law is accompanied by the absorption of heat (cooling) and expansion in volume.
Example 4
Ethanol (bpt. 78.50C) and tetrachloromethane (bpt.76.80C) form an azeotropic mixture of boiling point 65.00C.
(i) What is meant by azeotropic mixture? (2marks)
(ii) Draw a boiling point diagram for the ethanol-tetrachloromethane mixture (2 marks)
(You will notice that ethanol and tetrachloromethane mixtures deviate positively because the boiling point of the azeotrope is lower than that of either component; the boiling composition curve for a mixture that shows positive deviation is required)

(iii) Explain why ethanol and tetrachloromethane form an azeotropic mixture (2 marks)
The forces of attraction between like molecules (i.e. ethanol molecules or tetrachloromethane molecules) are stronger than those between unlike molecules (ethanol -tetrachloromethane molecules probably because tetrachloromethane interferes with the formation of hydrogen bond in ethanol while ethanol disrupts the van der Waal forces of tetrachloromethane. Therefore, molecules of each liquid in the mixture tend to escape into the vapor phase more easily than those of pure components. This makes the total pressure of the mixture higher than that expected from Raoult’s law.
Negative deviation
Negative deviation from ideality is said to occur when the vapor pressure of the mixture is lower than what it would be expected from calculations of Raoult’s law.

Causes of negative deviation
Unlike molecules interact (attract) with one another more strongly than like molecules do.
i.e A↔B > A↔A or B↔B
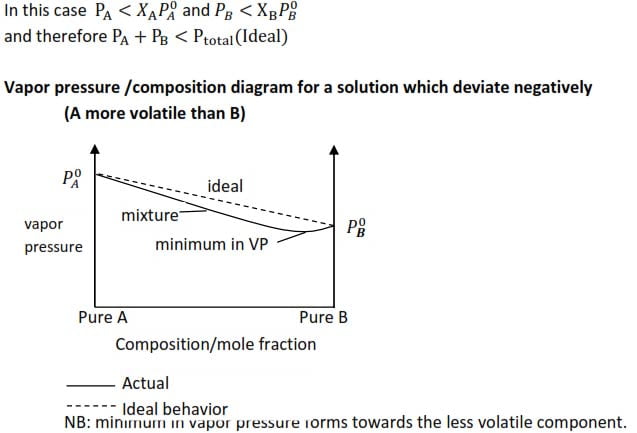
NB: minimum in vapor pressure forms towards the less volatile component.
An example of mixtures showing negative deviation are
(i) propanone (CH3COCH3) and chloroform (CHCl3);
(ii) water and hydrochloric acid.
Usually, mixtures showing negative deviation are obtained by mixing two polar compounds. The negative deviation, in this case, is due to hydrogen bonding between CHCl3 and CH3COCH3

Mixtures that deviate negatively from Raoult’s law form azeotropes with boiling points higher than those of either of their components.
Boiling point versus composition for a mixture that deviates negatively at constant pressure
(A more volatile than B)

The formation of a solution that deviates negatively from Raoult’s law is accompanied by the evolution of heat and contraction of volume.
Reasons why azeotropes are not compounds
- Can be separated by physical means
- Their composition varies with pressure.
- Cannot be represented by a chemical formula
Methods of separating an azeotropic mixture
- Adding a substance that reacts and removes one component. e.g. CaO reacts and removes water from azeotrope of ethanol and water
- Distillation with a third substance e.g. benzene in rectified spirit
- Fractional crystallization
- solvent extraction
- adsorption
Example 5
(a) (i) State Raoult’s (3marks)
A mixture of ethanoic acid (bpt. 1180C) and pyridine (bpt. 1230C) show negative deviation from Raoult’s law
(ii) Draw the vapor pressure /composition curve for the mixture of ethanoic acid and pyridine and indicate the line for ideal behavior (4marks)
Solution
To draw the correct diagram, the following should be put into account
- ethanoic acid with lower boiling point has a higher vapor pressure
- The minimum boiling point occurs to the composition containing more pyridine (the less volatile) than ethanoic acid.
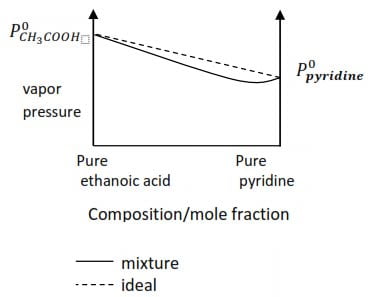
(iii) Explain the shape of the curve in relation to Raoult’s law
- the curve for the mixture lies below the line for ideal behavior
- this is because the attraction between the molecules of ethanoic acid and those of pyridine are greater (stronger) than those between ethanoic acid-ethanoic acid or pyridine –pyridine molecules
- pyridine molecules are held by weak van der Waal forces
- ethanoic acid molecules are associated with hydrogen bonds
- when ethanoic acid and pyridine are mixed, intermolecular hydrogen bonds are formed between ethanoic acid and pyridine molecules which are stronger than the bonds in pure or equal to those in pure ethanoic acid
- consequently, the escaping tendency of individual molecules of each component is reduced, leading to the reduced vapor of solution of the mixture
Watch this
Revision question? Download PDF below
Raoult’s law, fraction distillation

xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
xnxx porn
I’m eagerly awaiting your next post! Car & Motorbike
I’m always excited to see your new posts. Sports News
Check out the updated MBBS Cutoff Of Government Medical Colleges in Rajasthan to secure admissions.
Discover practical financial options for education with MBBS Fees Structure in Chhattisgarh.
Learn the key benefits of Raja Luck today.
Join the platform and activate benefits with the Diu Win Invite Code.
Discover flexible rental plans with innovative Server Rental in Gurgaon that meet your business requirements.
Download the supreme video gaming app today with bdg win app.
Experience seamless gameplay and interactive functions with 55 club app.
Enjoy hassle-free video gaming and quick navigation with the bdg win app.
Find essential Best Kitchen Tools And Gadgets that bring efficiency and design to your cooking area.
Unlike other converters, Onlymp3 Converter doesn’t need any installation, which is a huge plus.
Great insights about local transport! I found having company from Escort Service In Nainital practical for navigating the area too.
I can’t stop sharing my Joshital Love with my friends and family.
I had such an amazing time with Chennai Escorts last night!
Looking for a Dehradun Call Girl!.?.!? Make sure to pick securely.
Stay ahead with the **Latest News**, covering trending subjects in sports, tech, and organization.
The creativity in every short on https://www.youtube.com/@kpopbuzzindo/shorts is truly impressive!