
What assumptions of the kinetic theory of an ideal gas need to be modified to account for the behaviour of a real gas.

- The kinetic theory of an ideal gas makes several simplifying assumptions that don’t hold true for real gases, especially under conditions of high pressure and low temperature. Here are the key assumptions that need to be modified to better describe the behavior of real gases:
- Negligible Volume of Gas Molecules: The kinetic theory assumes that the volume of individual gas molecules is negligible compared to the volume of the container. In reality, gas molecules do occupy space, and their finite size becomes significant at high pressures, where the volume of the container is reduced.
- No Intermolecular Forces: The theory assumes that there are no attractive or repulsive forces between gas molecules. However, real gases experience intermolecular forces (Van der Waals forces), which affect their behavior. Attractive forces become significant at low temperatures, leading to deviations from ideal gas behavior.
- Perfectly Elastic Collisions: The theory assumes that collisions between gas molecules and with the walls of the container are perfectly elastic, meaning there is no loss of kinetic energy. In reality, some energy is lost in collisions, although it is often small enough to be negligible in many situations.
- Random Motion: While gas molecules do move randomly, the theory assumes complete randomness without considering the influence of intermolecular forces. In real gases, these forces can lead to non-random behavior, particularly under certain conditions.
To account for these deviations, the Van der Waals equation is often used as an improvement over the ideal gas law:
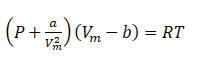
Where:
- P is the pressure of the gas.
- Vm is the molar volume of the gas.
- T is the temperature of the gas.
- R is the universal gas constant.
- a and b are empirical constants specific to each gas, accounting for intermolecular forces and the finite volume of gas molecules, respectively.
Please obtain free notes, exams and marking guides of Physics, chemistry, biology, history, economics, geography … from digitalteachers.co.ug website.
Thanks
Dr. Bbosa Science
CATEGORIES General
TAGS Dr. Bbosa Science
