
Alkanes (A-level organic chemistry)

Alkanes
This is the simplest honmologous series
General formula: CnH2n where n≥1
Examples


Compounds that contain the same molecular formula but different structural formulae like butane and 2-methylpropane are called isomers.
Definitions.
Isomerism is the existence of compound with the same molecular formulae but with different arrangements of atoms in a compound.
Types of isomerism
- Structural isomerism
- Optical isomerism
(a) Structural isomerism
Here compounds differ in the arrangement of atoms in a compound.
(i) Chain isomerism:
compound have the same molecular formula but with different arrangements of atoms in the chain.
Chain isomerism is common to alkanes where isomers differ in the arrangement of atoms in the chain
Example


(ii) Functional isomerism
Compounds have the same molecular formula but with different functional groups such as alcohols and ether.
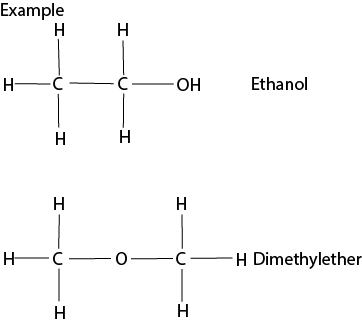
(iii) Positional Isomer:
The isomers have the same molecular formula, same functional group but different positions of the functional group on a molecule. Examples
CH3CH2CH=CH2 but-1ene
CH3CH=CHCH3 but-2-ene
(b) Stereoisomerism
Compounds have the same molecular formula, the same functional group but with different arrangements of atoms in space.
(i) Geometrical isomerism: atoms,
molecules are arranged differently about a double bond:
Example

(ii) Optical isomers:
Compounds have the same molecular formula, the same functional group but differ in optical properties towards plane-polarized light. Those that rotate light towards the right are called Dextro isomers and those that rotate light towards the left are called Levo isomers.
Nomenclature of alkanes
(a) Straight chain isomers
Straight chain isomers are named according to the number of carbon atoms in the chain.
The names of the first ten straight-chain isomers are given below

An alkyl group
is an alkane with one hydrogen atom removed
Examples
| CH4 Methane | CH3– | methyl group | |
| CH3CH3 Ethane | CH3CH2– | Ethyl group | |
| CH3CH2CH3 Propane | CH3CH2CH2– | Propyl group | |
| CH3CH2CH2CH3 Butane | CH3CH2CH2CH2– | Butyl group |
Because alkyl groups do not have chemical properties, they are generally represented by a letter R,
(b) Naming branched alkanes
(i) Determine the number of carbon atoms in the longest carbon chain that contains the branch.
(ii) Number the carbon atoms from the side nearest the branch. Example

(iii) If there more than one similar alkyl group on the longest chain; use di, tri, tetra to indicate the number of such groups on the main chain.

Note that
- This name implies that there is a methyl group attached to carbon 2 and another to carbon 3 of the butane.
- When writing the name of an organic compound, a coma (,) is placed between figures and a dash (-) between a figure and a letter.
(i) If different branches name them alphabetically
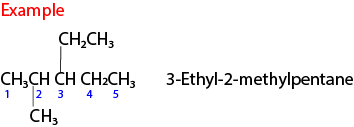
Note that
“E” for ethyl group comes before “M” for methyl group in the alphabets.
(v) If branching occurs equal distance from either side, choose a name that gives the least sum of combinations of numbers.
Example

2,2,3-trimethylbutane is the correct name because it gives the least sum for the combination of numbers. Exercise
Name the following compounds

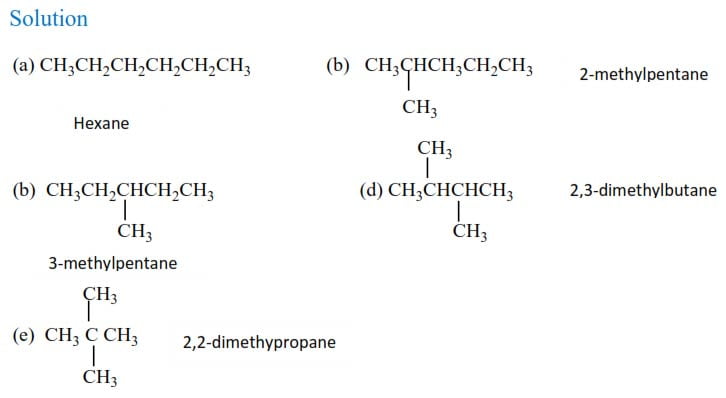
Physical properties of alkane
- they are insoluble in water
- they are soluble in organic solvents
- they range from gases to liquids to waxy solids
Chemical properties
1. They burn in air to produce carbon dioxide, water, and heat. Due to the production of heat, they are used as fuel.
Example
CH4 + 2O2 → O2 + 2H2O + heat
2. Chlorination: they react with chlorine in the presence of sunlight or u.v-light to produce chlorinated alkanes.

Mechanism
A mechanism is a step by step pathway followed by a reaction from the reactant to the products.
The following are steps followed in the chlorination of methane
- Chlorine molecules dissociate into atoms with an unpaired electron. Atoms or molecules with unpaired electrons are called free radicals. Free radicals are indicated by a dot on the atom that posses unpaired electron

2. Chlorine radical attacks a methane to produce hydrogen chloride and a methyl radical.

3. Methyl radical reacts with a chlorine molecule to form chloromethane and chlorine radical
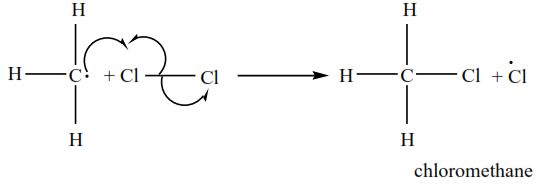
4 Chlorine radical attacks chloromethane to produce hydrogen chloride and chloromethyl radical.

5. Chloromethyl radical reacts with a chlorine molecule to form dichloromethane and chlorine radical

6. Chlorine radical attacks dichloromethane to produce hydrogen chloride and a dichloromethyl radical.

7. Dichloromethyl radical reacts with a chlorine molecule to form trichloromethane and chlorine radical

8. Chlorine radical attacks a trichloromethane to produce hydrochloride and a trichloromethyl radical.

9. Trichloromethyl radical reacts with a chlorine molecule to form tetrachloromethane and chlorine radical

Terminating steps
Meanwhile, the radicals may combine to produce molecules. The reactions where radicals react to produce molecules are called terminating steps i.e, they prevent the reaction from continuing.
Some of the terminating steps are:-
- Chlorine radical + chlorine radical produces chlorine molecule

2. Methyl radical + methyl radical produces ethane

3. Methyl radical reacts with chlorine radicals to form chloromethane and so on.

Sources of alkanes
- Petroleum product
- Biogas
Biogas is produced by anaerobic decomposition of organic matter (such as cow dung, plant remains, feces) in presence of water. The main component of biogas is methane.
Laboratory preparation
- By coupling reaction of alkyl halides in the presence of sodium and dry ether. For instance chloromethane couple with chloromethane to form ethane

2. By reduction of alkenes
Example

3. Reduction of alkynes
Example

4. Reduction of alcohols Example

5. Reduction of carbonyl compound Example

6. By reduction of carboxylic acid Example

7. By cracking long alkane
Cracking is the breakdown of long-chain hydrocarbons into short alkanes.
Cracking may be catalytic where a catalyst is used or thermal when the heat is used.
Watch this
Sponsored by The Science Foundation college +256 753 80 27 09
Compiled by Dr. Bbosa Science
Thank you for choosing Dr. Bbosa Science

Perfect and impossible becomes possible
Good for studying
What a good study platform
Real clear internet site, thanks for this post.
Hmm is anyone else encountering problems with the images on this blog loading?I’m trying to find out if its a problem on my end or if it’s the blogAny responses would be greatly appreciated
very nice
You’ve got a unique perspective, and I love it! Industrial & Scientific
You have a gift for explaining things clearly. cgnn
amox-clav 500 Chapter 1 discusses new trends in drug delivery areas via bioactive hybrid nanowires
You have a gift for making things memorable. Tech News
Learn about advanced healthcare education at Top MBBS Colleges in Kerala.
Enhance your gaming skills and enjoy endless fun with Raja Luck Game.