
Explain the variations of melting points of chlorides of period 3 elements

Physical properties of chlorides of period 3 elements
These are formed by reacting the elements with chlorine gas and the physical properties of their chlorides are shown in table 3.
Table 3 The chlorides of period 3 elements
| ELEMENTS | Na | Mg | Al | Si | P | S | Cl |
| CHLORIDES | NaCl | MgCl2 | AlCl3 | SiCl4 | PCl5 | S2Cl2 | Cl2 |
| BONDING | Ionic | Ionic | Covalent | Covalent | Covalent | Covalent | Covalent |
| STATES | Solid | Solid | Solid | Liquid | Solid | Liquid | Gas |
| MP\0C | 808 | 714 | 192 | -68 | 160 | -76 |
Explanation of melting points
NaCl and MgCl2 have high melting points due to the strength of the ionic bonding. AlCl3 has a fairly high melting point because in the solid state, it consists of Al2Cl6 molecules and not simple AlCl3. These molecules are produced through dative bonding between Al and Cl in the Al2Cl6 molecules.
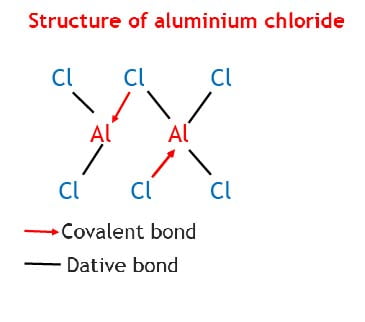
SiCl4 and S2Cl2 are liquids and consist of respective simple SiCl4 and S2Cl2 molecules and in the solid state the molecules are held by weak van der Waals force which explains their very low melting points. PCl5 a pale yellow solid has a fairly high melting point because the solid undergoes partial ionization.
2PCl5 ↔ PCl4+ + PCl6–
Please Subscribe to promote this website. Subscription is free
Share with a friend
We value your comments
Thank you so much

Thank you very much sir… We appreciate what you do and you have helped us alot.. Lord bless you.. We love you
I’ve improved in the profession coz I can now gather more information about my teaching subjects. Thx very much
I’m looking forward to your next post! Stationary
I always leave your blog feeling inspired. Barcelona News
Get reliable updates through the MBBS Cutoff Of Government Medical Colleges in Jharkhand.
MBBS Admission Through Management/Nri Quota in Madhya Pradesh offers a faster route to medical education.
Find out the latest trends in Raja Luck.
Download the newest version of GB Whatsapp with advanced features and improved security.
Eliminate downtime and increase efficiency with safe Server Rental in Chennai.