
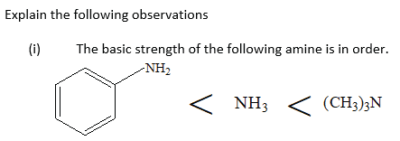
Explain why phenylamine is a weaker base than ammonia and ammonia is a weaker base than dimethylamine

Solution
Trimethylamine is a stronger base than ammonia because the methyl groups donate electrons to the nitrogen atom. This increases the density of alone pair of electron on the nitrogen atom on dimethylamine and its ability to attract a proton from water and form hydroxyl ions.
Phenylamine is a weaker base than ammonia because the phenyl group pulls electrons from the nitrogen atom. The reduction of electron density of alone pair on the nitrogen atom reduces the ability of the lone pair to attract a proton from water and form hydroxyl group
Please Subscribe to promote this website. Subscription is free
Share with a friend
Your comment is valuable
Thank you so much
CATEGORIES Organic chemistry
TAGS Dr. Bbosa Science

I’m looking forward to more from you! Clothes & Accessories
Your writing is top-notch. TamilBlasters Com