
Explain the variations of boiling points of chlorides of period period 3 elements

Boiling points of chlorides of period 3 elements
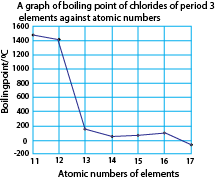
The table below shows the boiling points of the chlorides of period 3 elements.
| Element | Na | Mg | Al | Si | P | S | Cl |
| Atomic number | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Formula of the chloride | NaCl | MgCl2 | AlCl3 | SiCl4 | PCl3 | S2Cl2 | Cl2 |
| Boiling point (oC) | 1465 | 1418 | 180 | 57 | 76 | 136 | -35 |
A graph of boiling points of the chlorides against atomic number of the elements.
Explanation
NaCl and MgCl2 have high boiling points due to strong the ionic bonding.
Aluminium chloride, silicon chloride, phosphorus chloride, disulphur dichloride and chlorine have low boiling points because their molecules are held by weak molecular forces.
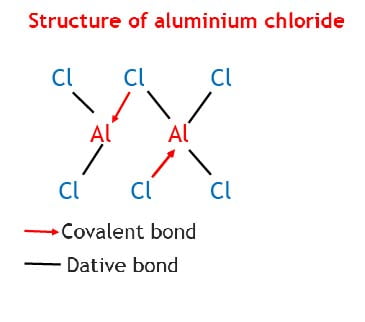
AlCl3 has a fairly high boiling point because in the liquid state, it consists of Al2Cl6 molecules and not simple AlCl3. These molecules are produced through dative bonding between Al and Cl in the Al2Cl6 molecules.
Disulphur dichloride has relatively high boiling point due to its high molecular mass that increases the strength of the molecular forces
Please Subscribe to promote this website. Subscription is free
Share with a friend
We value your comments
Thank you so much

Digital teachers have helped me to over hardship in A-level thanks for the effort you invest in provide a good service to students the almighty bless the admin abundantly
I will really be thankfull for your service sir
The best
I really appreciate your efforts
Hmm іt looқs liҝe your website ate my first comment (it was super
long) so I guesѕ I’ll just sum it up what I wrote and say, I’m
thoгoughly enjoyіng your Ьlog. I too am an aspiring blog writer but I’m still new
to the whole thing. Do you have any tips for
newbie blog writers? I’d really apprecіate it.
Thanks for being such a guide. Electronics
Your blog is a breath of fresh air. 500 ka redeem code
Gain insights into the Top MBBS Colleges in Andhra Pradesh, shaping the next generation of medical professionals.
Streamline your application process with MBBS Direct Admission in Sikkim.
Download the Raja Luck App to enjoy seamless gaming on your mobile device.
Get all the details about Raja Luck for better knowledge.
Unlock exciting gaming opportunities with Raja-Luck and experience thrilling lotto video games.
Release AI, ML, and cloud-based applications with Server Rental in Delhi.